- Comparison of atezolizumab plus bevacizumab and lenvatinib for hepatocellular carcinoma with portal vein tumor thrombosis
-
Jeayeon Park, Yun Bin Lee, Yunmi Ko, Youngsu Park, Hyunjae Shin, Moon Haeng Hur, Min Kyung Park, Dae-Won Lee, Eun Ju Cho, Kyung-Hun Lee, Jeong-Hoon Lee, Su Jong Yu, Tae-Yong Kim, Yoon Jun Kim, Tae-You Kim, Jung-Hwan Yoon
-
J Liver Cancer. 2024;24(1):81-91. Published online January 19, 2024
-
DOI: https://doi.org/10.17998/jlc.2023.12.25
-
-
1,012
Views
-
134
Downloads
-
 Abstract Abstract
 PDF PDF Supplementary Material Supplementary Material
- Background/Aim
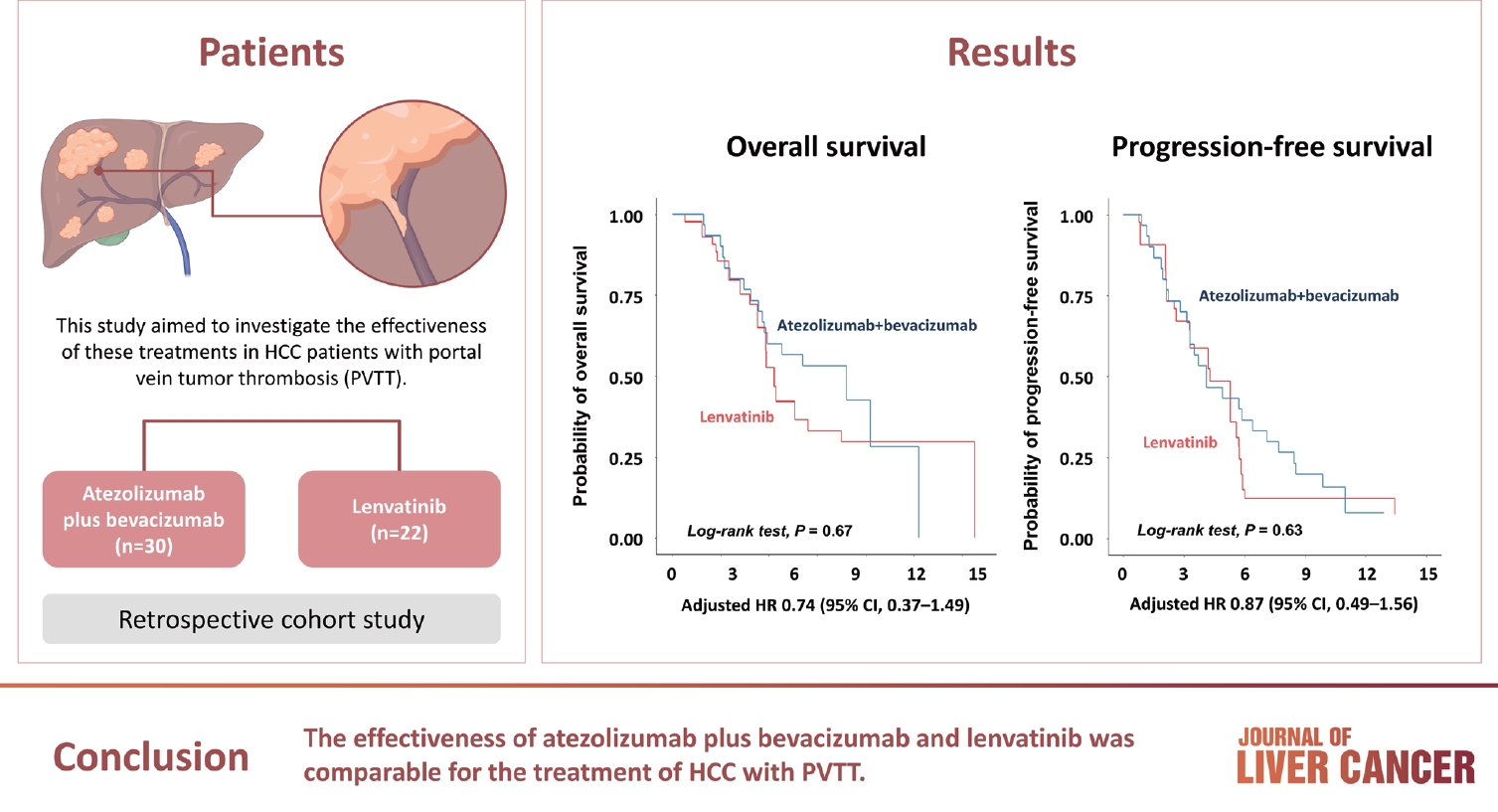
Atezolizumab plus bevacizumab and lenvatinib are currently available as first-line therapy for the treatment of unresectable hepatocellular carcinoma (HCC). However, comparative efficacy studies are still limited. This study aimed to investigate the effectiveness of these treatments in HCC patients with portal vein tumor thrombosis (PVTT).
Methods
We retrospectively included patients who received either atezolizumab plus bevacizumab or lenvatinib as first-line systemic therapy for HCC with PVTT. Primary endpoint was overall survival (OS), and secondary endpoints included progressionfree survival (PFS) and disease control rate (DCR) determined by response evaluation criteria in solid tumors, version 1.1.
Results
A total of 52 patients were included: 30 received atezolizumab plus bevacizumab and 22 received lenvatinib. The median follow-up duration was 6.4 months (interquartile range, 3.9-9.8). The median OS was 10.8 months (95% confidence interval [CI], 5.7 to not estimated) with atezolizumab plus bevacizumab and 5.8 months (95% CI, 4.8 to not estimated) with lenvatinib (P=0.26 by log-rank test). There was no statistically significant difference in OS (adjusted hazard ratio [aHR], 0.71; 95% CI, 0.34-1.49; P=0.37). The median PFS was similar (P=0.63 by log-rank test), with 4.1 months (95% CI, 3.3-7.7) for atezolizumab plus bevacizumab and 4.3 months (95% CI, 2.6-5.8) for lenvatinib (aHR, 0.93; 95% CI, 0.51-1.69; P=0.80). HRs were similar after inverse probability treatment weighting. The DCRs were 23.3% and 18.2% in patients receiving atezolizumab plus bevacizumab and lenvatinib, respectively (P=0.74).
Conclusion
The effectiveness of atezolizumab plus bevacizumab and lenvatinib was comparable for the treatment of HCC with PVTT.
|